Abstract
Background: Success of alternative donor stem cell transplantation (SCT) for acquired and inherited bone marrow failure (BMF) syndromes has previously been limited by significant risks of severe graft versus host disease (GvHD) and graft failure, particularly for patients who lack fully HLA-matched donors. The development of post-transplant cyclophosphamide (ptCy)-based haploidentical donor SCT has mitigated but not eliminated these risks, and the relatively slow engraftment seen with ptCy bone marrow grafts may not be ideal given high rates of infectious disease and bleeding concerns in patients with BMF. Partial T cell depletion of mobilized peripheral stem cell (PSC) grafts can greatly reduce risks of graft versus host disease while facilitating rapid and robust engraftment by providing a high stem cell dose. We previously reported excellent outcomes with minimal GVHD and no graft rejection using peripheral stem cell transplant (PSCT) combined with ex vivo CD3 +/CD19 +depletion and low dose CD3+ T cell addback from matched unrelated donors (MUD) and mismatched unrelated donors (MMUD) in pediatric patients with BMF. Here, we describe the use of selective ex vivoT cell receptor (TCR)αβ +/CD19 +depletion of mobilized PSC from MUD and MMUD for pediatric patients with BMF, which has the advantage of retaining TCRgd +T cells which may help facilitate engraftment and decrease infections.
Methods: We report the outcomes of 26 pediatric patients with BMF (excluding MDS-defining clonal evolution) who underwent MUD/MMUD PSCT with TCRαβ +T cell/CD19 +depletion using CliniMACS at The Children's Hospital of Philadelphia from 2017 to 2021. Patients were enrolled on a prospective clinical trial for patients with BMF (NCT03047746, n=21)or on an expanded access study (NCT03145545, n=5). Conditioning regimens consisted of thymoglobulin (9mg/kg), cyclophosphamide (100mg/kg), fludarabine (150mg/m 2), and low dose TBI (200-300 cGy) for patients with acquired BMF disorders, thymoglobulin (9mg/kg), busulfan (PK-adjusted), fludarabine (150mg/m 2), and thiotepa (10mg/kg) for patients with single lineage BMF or thymoglobulin. One patient with Fanconi Anemia received thymoglobulin and fludarabine, with reduced dosing of busulfan and cyclophosphamide. Patients undergoing MSD-BMT for BMF over a similar time period served as a comparison group for engraftment kinetics, rates of GVHD, donor chimerism, immune reconstitution, and overall survival.
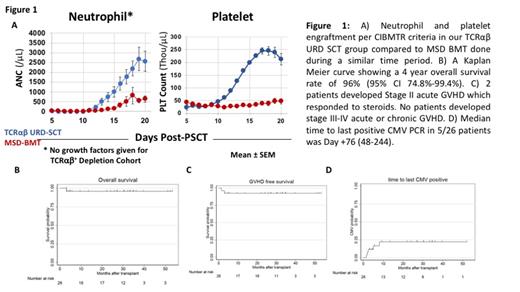
Results: Subjects included 18 with severe acquired aplastic anemia (SAAA), 4 with SAAA and concurrent paroxysmal nocturnal hemoglobinuria (PNH), 2 patients with acquired BMF not otherwise specified, 1 patient with DBA, and 1 patient with Fanconi Anemia. 11 patients with SAAA underwent SCT as initial therapy while 11 patients had SCT after failing previous medical therapies. Median age at diagnosis was 10.3 years (0.1-20.6) and at transplant 11.1 years (0.9-21). HLA match of unrelated donors was either 10/10 (n=15), or 9/10 (n=11). Median CD34 +and TCR αβ +T cell dose was 12.0x10 6cells/kg (3-22.6) and 0.1x10 5cells/kg (0.0-4.2). Median times to neutrophil and platelet engraftment per CIBMTR criteria were 15 days (10-22) and 15 days (13-19), respectively, both significantly earlier than engraftment following MSD-BMT (Fig 1a). At a median follow-up of 727 days (39-1498), 25 of 26 patients are alive with resolved hematologic disease (Fig 1b). One patient with SAAA+PNH who failed prior IST achieved trilinear engraftment without GVHD, but died on Day+95 due to acute disseminated toxoplasmosis. No patients exhibited immunologic graft rejection. 2/26 patients had grade II acute GVHD that responded to steroids and none developed Grade III-IV acute or chronic extensive GVHD (Fig 1c) CMV viremia/reactivation occurred in 5 subjects, all responding to antiviral pharmacotherapy, and none developed end-organ CMV disease (Fig 1d). One patient developed recipient-derived EBV post-transplant lymphoproliferative disorder requiring multimodal treatment. Only one patient developed BK cystitis. Total peripheral chimerism exceeded 90% in all patients. Immune reconstitution kinetics were similar to that seen in MSD-BMT.
Conclusion: MUD/MMUD PSCT with TCRαβ +T cell/CD19 +depletion in patients with BMF enables rapid, durable engraftment with minimal risk of GVHD and immunologic graft rejection.
Monos: Omixon: Consultancy, Patents & Royalties. Grupp: Jazz Pharmaceuticals: Consultancy, Other: Steering committee, Research Funding; Novartis, Adaptimmune, TCR2, Cellectis, Juno, Vertex, Allogene and Cabaletta: Other: Study steering committees or scientific advisory boards; Novartis, Kite, Vertex, and Servier: Research Funding; Novartis, Roche, GSK, Humanigen, CBMG, Eureka, and Janssen/JnJ: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal